VOC – Volatile organic compound – What are they?
VOCs: nature, classes of compounds and environmental effects!
The goal of this article is to provide you information on the Volatile Organic Compounds (VOC), from three different perspectives:
- What are they? To answer that question we’ll show you the meaning of VOCs.
- What are the classes of compounds that characterize VOCs? What are the classes of molecules that fall into this category and where they are used.
- How they behave in the atmosphere? In other words, what effects these species have on chemical balance of the atmosphere and how they are related with the issues of health and pollution.
What are VOCs?
Inside the definition there are the words “organic” and “volatile”. What is meant by these terms?
- Organic: This word shows that VOCs are based on carbon chemistry (organic chemistry). The species that we discuss have functional groups (groups of atoms) that govern their chemical and physical behavior and responsiveness.
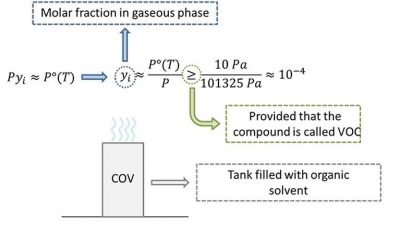
- Volatile: This term aims to highlight the fact that VOCs have a marked tendency to transit in vapor phase. In particular, the Italian law defines as VOC those organic compounds that to 293,15 K are located in gas phase, or that, if they are in liquid phase, have a vapor pressure greater than 0.01 kPa. In other words, leaving a tank containing an organic substance in contact with the atmosphere (at 20 ° C), the substance is defined VOC if in the gas phase is measured a concentration greater than 100 ppm.
In summary, VOCs are organic molecules that can easily be found in gaseous environments. Let’s see what are the main categories of molecules that respect the definition given.
What are the classes of compounds that characterize VOCs?
Before to distinguish the different types of molecules that characterize VOCs, it is useful to note that these molecules can come from natural sources (biogenic origin), human processes (anthropogenic origin), or from both sources.
The majority of natural compounds are derived from vegetables. These include methane (resulting from an anaerobic decomposition process of organic substrates), a class of unsaturated hydrocarbons called terpenes and other classes of organic compounds (such as esters, aldehydes, ketones and peroxides).
The man-made compounds instead, come mainly from industrial processes and products. This is the category of greatest interest for us so let us examine it in more detail. Among the VOCs produced by human processes we can include:
- Aliphatic hydrocarbons: They are chemical species containing carbon and hydrogen, linked together by single bonds only. They constitute a significant fraction of oil and are used extensively in the field of fuels.
- Alkenes: a kind of hydrocarbon containing double bonds. They derived from production processes of the petrochemical industry and are very important intermediate for the synthesis of many compounds.
- Aromatic hydrocarbons: They are very stable molecules used in many processes and products (paints, varnishes, glues, lacquers …)
- Aldehydes: They are the molecules partially oxidized, widely used in the chemical and agricultural sector (fungicides, insulation, germicides, resins, disinfectants …)
- Alcohols: are widely used as solvents or as intermediates in the chemical processes of high importance. Lately they are covering an increasingly part of automotive fuels.
- Ethers: They are used in specific contexts, where they can be found as air contaminants. For example, THF (tetrahydrofuran) is used as an industrial solvent, while the MTBE (methyl-tertbutil-ether) is widely used as anti-knock in green gasoline.
- Halogenated organic compounds: In industrial applications are used a large quantities of halogen derivatives, both aliphatic and aromatic. Compounds are typically volatile, hydrophobic and toxic, and are widely used as pesticides and refrigerants.
- Organic sulfur compounds: the majority of these compounds do not constitute a serious problem for the environment, but at the local level can be harmful. Human activity produces them through the treatment of animal waste, water sewage and in oil refining processes.
- Organic nitrogenous compounds: This class covers a large number of chemical species (amines, amides, nitriles …) which are used in a wide range of areas. Among these, there are the production of dyes, pharmaceutical chemistry, photography and production of rubbers and polymers.
What effect do they have?
Now that we have developed a greater awareness of what are VOCs and what classes of molecules characterized it, it is useful to analyze the possible effects within the atmosphere.
In general it can be said that each pollutant has a characteristic time of permanence in the atmosphere, linked to its chemical-physical properties. The greater this time turns out to be, the more the pollutant can be dispersed in the atmosphere for work of winds and currents, altering the chemical balance of the atmosphere itself. For example, many VOCs affect the balance of methane (CH4), prolonging his stay in the atmosphere and thus contributing to the increase of the greenhouse effect.
It is also important to note that many VOCs are dangerous to humans and animals. Benzene, for example, is a carcinogenic compound characterized by high volatility. Formaldehyde is another toxic compound produced in high quantities, and commonly employed in many production processes. Even the halogen compounds have high volatility and toxicity; moreover, being tendentially hydrophobic, they can accumulate in the body.
Each chemical compound deserves a deep analysis, but that is outside the objectives of this article. We’ve seen what VOCs are, by which classes of compounds are characterized and their potential danger for the atmosphere and for living beings.
So, how can we act?
At this point, a spontaneous question arise: how is it possible to act in industrial processes to minimize the amount of VOCs released into the atmosphere? In answering this question it is important to take into account the potential flammability of these substances: sometimes turns out to be necessary to design ATEX plants.
The separation mechanisms and devices aimed at this goal will be the subject of a new article. To satisfy your curiosity, I invite you to examine immediately the equipment designed by Tecnosida® for this particular category of pollutants:
- Chemsorb® (activated carbon filter)
- WETCLEAN (scrubber based on wet abatement)
- Oxither (thermal oxidizer both direct flame, recuperative and regenerative)
- BIOCLEAN (biofilter)
Find out our case history to discover their application!
See you soon with new interesting articles!