Combustible dust hazard: chemical-physical characteristics, explosion class, KST values and dust explosion pentagon
A detailed study on the dust explosions in the factories
In some previous articles we have examined in depth the ATEX case, analysing the European directives which define the required needs for the devices and the protection systems to use in potentially explosive areas. In the article “Industrial ventilation systems for potentially explosive atmosphere” we had already given a complete review of the components to be used in the aspiration systems installed in these areas, instead in this article we will give an answer to the question “where do the dust explosions come from?”, examining the conditions that involve the explosion of combustible dusts and their physical parameters.
Definition and historical background of the dust explosion
A dust explosion can be defined as a sort of damaging event caused by a violent chemical reaction of a combustible dust, which occurs in specific situations.
The first news on a dust explosion dates back to 1785, when a flour explosion was reported in a bakery in Turin. Nowadays, the explosions affect the manufacturing industries and the work processes which involve the use of dust materials. This event often occurs: according to some studies, a dust cloud explosion would take place every day. In most cases, these events don’t have devastating consequences. However, sometimes they can cause widespread damage that can bring about the complete or partial closing of the activities involved. Below we have reported some cases as examples:

- 1999 – explosion of coal dusts in a Ford Motor Company’s plant in Michigan. This event caused the death of 6 people and 36 people were wounded.
- 2000 – an explosion of pyrophoric aluminium dust took place in an Italian metal polishing factory. During the grinding and surface finishing processes, fine metal particles were released and ignited in contact with air causing a very exothermic and explosive reaction. About ten workmen were burned and the facilities were damaged;
- 2008 – in the sugar refinery of the Imperial Sugar (Georgia) a bearing put under the sugar silos overheated, activating the sugar dust mixture. The explosion spread in the packaging area causing the death of 14 people and 40 people were wounded.
- 2017 – in a farm in the South of Indiana a grain silo fell down causing a great explosion of grain dusts and damage to the facilities.
Explosive Dust Explosion Pentagon
After the definition of the dust explosion and its effects, now it’s time to understand how these events arise.
In order to do this, we must introduce another important element: the Dust Explosion Pentagon. This one represents the five requirements for a dust explosion, i.e.
- Oxidant (basically it is the oxygen in the air);
- Ignition source;
- Confinement;
- Combustible dust;
- Dispersion of dust.
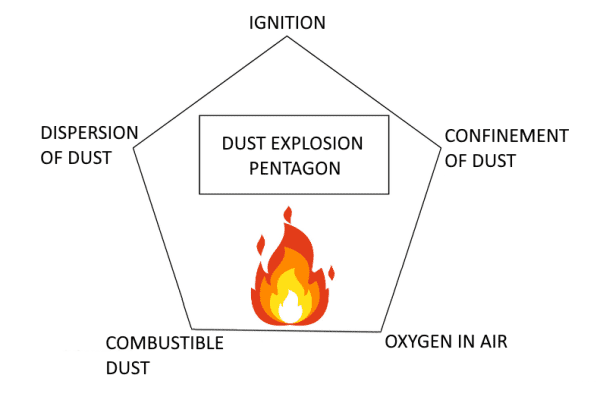
We have already run into some of these elements in our article about the VOC’s heating oxidation, when we talked about the fire triangle and the flammability conditions of liquid and gas fuels. In order to cause explosions, they require the simultaneous presence of an ignition source, fuel and a suitable quantity of oxygen. On the other hand, in order that the explosive dust can explode, it must have two other features: dispersion ease and ability to create a suspension with air in confinements.
Generally, we can say that any combustible dust, originating from oxidised metal materials (aluminium, iron etc…), natural organic materials (sugar, cereals, etc…) or synthetic substances (pesticides, plastics materials, etc…), can potentially explode.
The explosive feature depends on the physical-chemical properties of the dust and on other situations connected to the environment. In the next paragraph we will analyse the main parameters which define the dust level of danger, thus of the explosions.
Physical-chemical properties of the combustible dust and explosiveness parameters
These are the main parameters that define the dust level of danger: Kst, Pmax, MIE, MIT, LEL and UEL. Let’s analyse them:
Combustible dust Kst values and Pmax
The Kst index, together with the Pmax index (maximum explosion pressure of a given powder – its unit of measurement is bar), is the date that allows the risk of explosion connected to a plant or a process to be evaluated and allows us to understand how destructive a given powder is in case of an explosion. Different classification systems have been adopted, in order to compare the explosive violence of dust. One of the most famous systems refers to the Bartknecht classification, which divides the combustible dusts in four danger classes, according to the Kst index:
| Explosiveness class | Kst [bar⋅m/s] with primer from 10 kJ | Kst [bar⋅m/s] with primer from 10 J | Type of explosion |
| St 0 | 0 | 0 | None |
| St 1 | >0-200 | >0-100 | Weak |
| St 2 | >200-300 | >100-200 | Strong |
| St 3 | >300 | >200 | Very strong |
Kst and Pmax indexes are the main parameters to be used in the safety panels‘ assessment or in their elimination systems. As a matter of fact, as soon as the pressure suddenly increases, you will have to use wider panels or to create a more resistant pressure filter.
Limits of explosion (LEL – UEL)
The LEL (Lower Explosion Limit) and the UEL (Upper Explosion Limit) represent the explosiveness limits and they are respectively the concentration of flammable material in the air under and above the explosion range. These limits are measured mixed with air.

Minimum Ignition Energy (MIE)
The MIE (Minimum Ignition Energy) is the energy that is able to ignite the tested dust, under specific test conditions. When we speak about the explosive dust we must consider that the MIE is connected to the particle size: the fine particles usually have lower ignition energies than the rough ones.
Minimum ignition temperature (MIT)
The MIT (Minimum Ignition Temperature) represents the minimum temperature in which the dust can ignite. In this case, we must specify that the dust, unlike gas and mists, have two ignition temperatures: one is related to the dust cloud and the other refers to the dust level ignition (LIT)
Combustible dust list
In the table below we report a list of some combustible dusts examples with their approximate parameters previously described:
| Dust | Kst (bar.m/s) | Pmax (bar) | LEL (g/m3) | MIE (mJ) | MIT (°C) |
| Aluminium | 620 | 5.8 | 60 | 10 | 560 |
| Sugar | 90 | 7.5 | 34 | 10 | 360 |
| Coal | 70 | 6 | – | – | 660 |
| Sulfur | 151 | – | – | 35 | 280 |
| Aspirin | 217 | 6 | 15 | 15 | 550 |
| Graphite | 71 | – | 30 | – | 600 |
| Fructose | 102 | – | 60 | 1 | 430 |
The data listed above are purely indicative and are subject to variations.
Physical parameters conditioning the dust explosiveness
In order to complete this article, we must add that the dust explosiveness is conditioned by several factors, such as the chemical composition, type of fuel, particles size, humidity, etc… Let’s analyse them:
Chemical composition and responsiveness
As soon as a dust explosion spreads, a reaction between dust particles and atmospheric oxygen occurs. The chemical nature of the dust and its calorific value influence the speed of the oxygen consumption. This last parameter is essential, because it sets the quantity of heat that can be released during the explosion.
Type and concentration of the oxidant
As we have seen, the oxygen is one of the most common oxidants. The oxygen’s increase in the ATEX atmosphere sharpens the combustion reaction, thus increasing the explosion rate. On the other hand, in order to stop the explosion, it is necessary to reduce the oxygen by mixing it with noble gases, such as nitrogen, carbon dioxide, etc…
Particle size
As soon as the dust particles size decreases, the contact area increases and the dust burns more easily. Besides, the micro-particles are more likely to disperse and remain suspended for a longer time. Even the explosion maximum pressure increases, as soon as the particle size decreases.
Humidity
Generally, we can say that the presence of humidity tends to reduce the explosiveness, because the dust particles become more cohesive and create some conglomerates, which are more difficult to disperse, thus their ignition is harder. However, this reaction is not valid for all the types of dust.
As we have previously seen, the explosive dust can be more or less dangerous, depending on their different chemical-physical properties and the external factors that can condition their explosion.
In order to avoid detrimental consequences, the plants used in the ATEX environments must be sized and organised according to the type of dust, the environmental factors and their standards.
If you need to examine in depth the subject matter and discover all the components to be used in the ATEX plants, feel free to visit our website or contact us to receive a professional and customised advice!