Oil mist: what are they? How to treat them?
Read our article and discover with us the genesis of oil mist and the principle of coalescence
Summary
Fog is a very fascinating phenomenon (except if you are driving). At the base of its formation are hidden a series of physical and chemical phenomena of great interest: let’s examine them to explain oil mist nature and abatement!
In particular, in this article we will analyze:
- What oil mist are? We examine what aerosol are and the concept of “colloid”. From these concepts you will be able to understand the properties of the oil mist.
- How do they form? What are mechanisms that can lead to the formation of oil mist in industrial contexts?
- How to treat them? We examine the physical phenomenon of coalescence
What are oil mists?
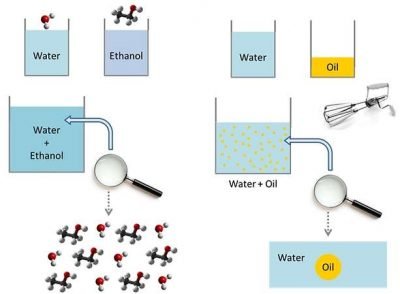
To explain what oil mists are, is necessary to introduce some preliminary concepts. We will examine them gradually, starting from an experiment. Imagine to take four different cans: two of them contain distilled water, while the other two contain, respectively, ethanol and oil. If you mix water and ethanol, you get a mixture that shows uniform characteristics. In other words, we have a mix of water molecules and ethanol ones. If there was a magnifying glass able to see the individual molecules, we would notice that the composition is identical in all parts of the solution.
So we have an homogenous mixture characterized by a single phase composed by the two molecules.
If you mix water and oil, happens something different.
In fact, while water and ethanol’s molecules are in harmony together, for water and oil molecules isn’t the same. Water and oil union doesn’t create an homogeneous mixture. This, chemically, is linked to the fact that water and ethanol have polar character, while oil is apolar. If you mix the two substances with a mechanical whisk, provided that water quantity is greater than oil, you go to create a set of oil droplets immersed in the aqueous solution. This is an heterogeneous mixture, characterized by two distinct phases.
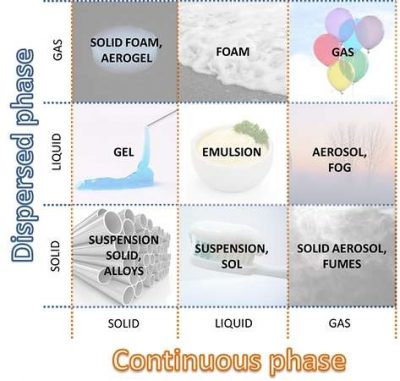
We focus on the heterogeneous mixture. In the specific case of the experiment, two liquid phases are mixed. Water is present in greater quantities so it behaves as a continuous phase, in which are dispersed the oil droplets (dispersed phase).
The question is: can you have a similar effect by considering solid and/or gaseous phases? The answer is yes, and the compounds created are called colloids.
A colloid is a mixture in which solid, liquid and/or gaseous phases are finely dispersed in another one. Typically the size of particles of the dispersed phase are between nanometer and micron.
According to the aggregation state, it’s possible to classify different types of colloidal systems. In this article we are interested in aerosols and mists: fine dispersions of liquid particles into a gaseous phase. Oil mist, in particular, correspond to fine air-borne oily droplets.
Now let’s speak about oil mists formation.
How does oil mists originate?
Typically oil mists come from processes that use fuel oils, lubricating oils, hydraulic oils or high temperature polymeric products. The question is to understand how these types of substances can form, from the liquid phase, a fine dispersions in the air.
The first important mechanism is spraying. If a fluid, maintained at high pressure, passes through a thin slit (nozzle), it tends to spray and to form fine droplets. This phenomenon can be created voluntarily (for example in combustion processes), or may happen in an undesirable way (for example in the cracking of a high pressure tank).
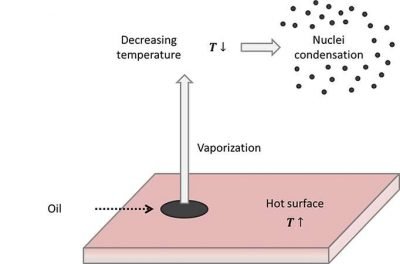
A second phenomenon, frequently present in plant field, is the vaporization and nucleation due to thermal increases. Thermal increments of a given substance are related to an average increase of molecules’ kinetic energy, that create an increased tendency to vaporize. If the types of oil before described contact with hot surfaces they tend again to vaporize. Then, if the oil present in the vapor phase meets a colder area, the value of oil vapor pressure decreases greatly, with consequent tendency to condensation. In gaseous phase we will have some condensation cores that create oil mists.
We analyzed what oil mists are and how they form. Now we can discover how we can remove them from gas streams!
How does oil mists collection work?
The removal of oil mist is performed through a physical principle known as coalescence.
What is the coalescence? Coalescence is the physical phenomenon through which the drops of a liquid, rather than the droplets of a gaseous substance, or the particles of a solid, are combined together to form larger drops. This principle is used in order to ” enlarge ” drops, so as to make them more easily to be removed from the gaseous stream. Since the oil mist airborne is a thermodynamically unstable system, they would tend, perhaps even after an infinite time, to separate from the effluent which are dispersed combining autonomously thanks to the spontaneous coalescence that are physically submitted. To explain this concept, we can consider the mixture of oil and water before mentioned.
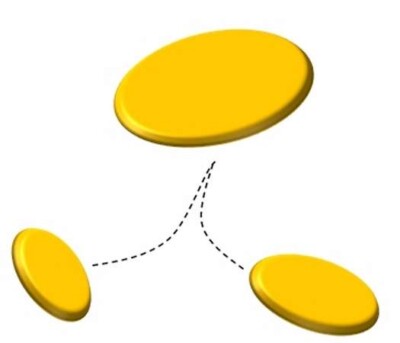
We can see that, if let’s shake vigorously the above mixture, forming a fine dispersion of oil droplets in water. It is also true that, if not more we mix the dispersion thus created, the oil droplets tend to coalesce and to form a single oil phase.
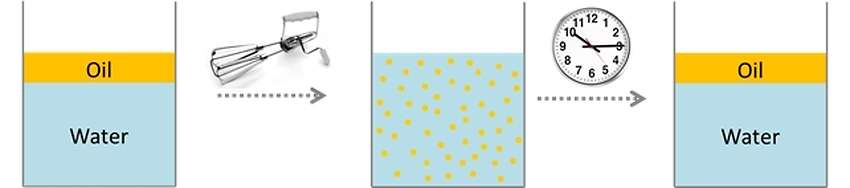
This fact provides us very important experimental information: water and oil tend to stay as far separate as possible. In more scientific terms, the two substances spontaneously tend to minimize their contact surface. The fact that the dispersion of oil in water is created, is only linked to the mechanical power that we introduce in the system, by agitation.
From here we deduce that the water / oil mixture is not thermodynamically stable. In other words, if you leave enough time to the mixture, the oil droplets tend to coalesce and to separate from the aqueous phase. The “stable” emulsions, commercially used, are in fact in most cases only stable in the kinetic level: stabilization mechanisms are introduced to make the kinetics of aggregation of the particles very slow.
At the base of these considerations, there is a physical quantity of extreme importance: the interfacial tension (γ). This physical quantity expresses the “energy cost” that must spend to create a surface between two different phases. Since create a separation surface is typically expensive in terms of energy, the two different phases tend to minimize their contact surface, separating.
In the industrial sector, however, we cannot wait forever to make this phenomenon occur naturally so Tecnosida® has developed a oil mist abatement system called OIL SCREEN.
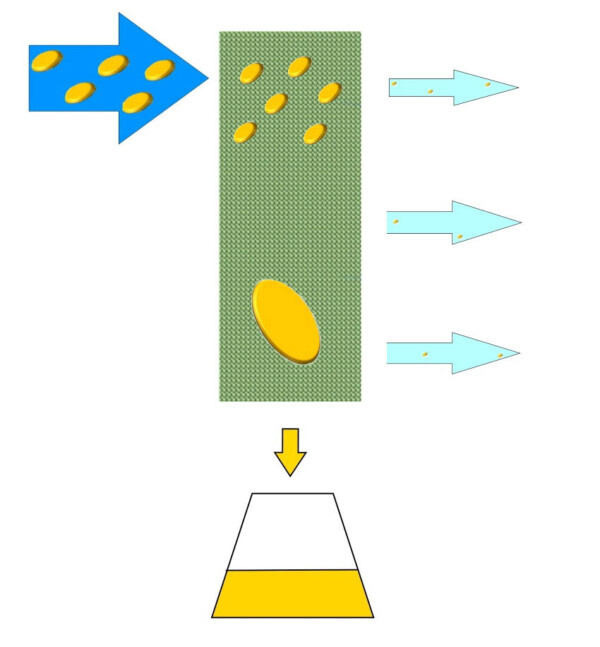
The OILSCREEN uses a special surface that promotes contact of oily droplets dispersed in order to promote the phenomenon of coalescence under a kinetic point of view. Once grouped together, these drops tend to fall down by gravity, towards the lower part of the filter where the oils are then collected for disposal.
The treated effluent is then aspirated by the fan and sent toward the eject stack purified by these oily substances. OILSCREEN make the most of its potential if the treated oils have a high viscosity and if the flow is free from dust. In fact, depending on possible other pollutants present in the stream to be treated, the system may require a pre-treatment.
Tecnosida®, in addition to providing design and construction to the filtering apparatus, also provides a cleaning service, maintenance and replacement of coalescing cartridges, so as to ensure over time the efficiency of the filtration.
We applied OILSCREEN with success in a wide variety of contexts, including:
- The field of thermal treatment of metals
- The high temperature treatment of synthetic polymers
- The packaging of foods
Contact us for more information!
See you soon with new interesting articles!