What is carbon monoxide (CO)? Which are its industrial uses, how it is produced?
Discover the characteristics of this chemical species and the problems arising from its involuntary production
What is carbon monoxide? How it is produced? What are its uses in the industrial world? Why, in some contexts, it is an unwanted contaminant species, against which action?
In this article we will examine together the interesting answers to these questions to better understand the role of this chemical species and the problems that can arise when you have a production unintended.
We start from the analysis of the first question.
What is carbon monoxide?
The carbon monoxide is a molecule in which a carbon atom (C) and an oxygen atom (O) are covalently linked between them. The properties of a molecule greatly depend on the nature of the bonds between atoms that make it up: why it is important to examine them?
Imagine you join two objects with a rope. Now imagine them together with two ropes in parallel, and then imagine them together with three ropes! Intuitively, you can see that the connection between the two objects is stronger the more they are the cords that bind them.
For molecules the reasoning is similar: if two atoms are linked by a single bond, double or triple, their connection becomes more and more stronger.
I’ll give you one example. Consider the air we breathe every day. The air turns out to be mainly composed of molecular nitrogen (N2, which composes about 79% of the air) and molecular oxygen (O2, which composes about 21% air). In Nitrogen molecule, the two nitrogen atoms are linked by a triple bond. In oxigen molecule, between the two oxygen atoms elapses a double bond.
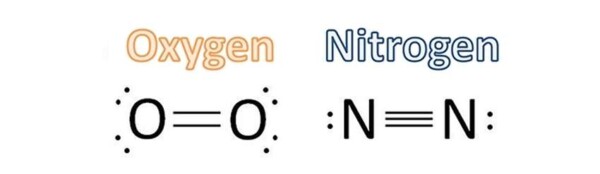
What has this to do with carbon monoxide? We use the analysis as a basis for understanding the type of this bond in this molecule.
Between the carbon and the oxygen is present a double bond or a triple bond? The answer can be double: neither, or both. What do you mean? The image clarifies the concept.
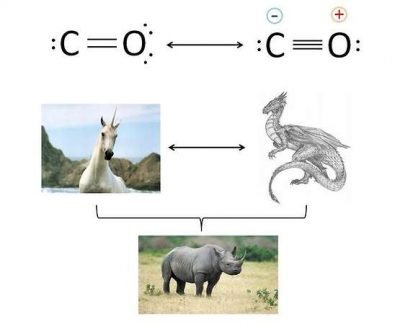
The carbon monoxide has two resonance structures limit. In other words, the present bond in this molecule can be described from two different configurations:
- In the first configuration, between C and O exists a double bond, and the two atoms have zero formal charge.
- In the second configuration the oxygen shares a further electronic doublet and between the two atoms there is a triple bond, with the presence of formal charges. In particular, the carbon has a formal negative charge and the the oxigen have a positive formal charge.
The actual structure of the CO is a mix between the two resonance limit structures, in which greater stability to the structure (experimentally, one with formal charges) provides the greatest contribution.
To better understand the concept, look at the bottom of the image. Neither the unicorn or dragon (resonance limit structures) exist, but their possible mix (the rhinoceros, the real structure) is an existing animal.
The link is neither double or triple, but it looks a lot more than three times. The carbon and oxygen atoms do not have net charges, but they still have some significant amount of charges.
Are these charges that affect the reactivity of the carbon monoxide. While the molecular nitrogen has a triple bond and neutral charge (elements which, in the presence, make it less reactive), the carbon monoxide has a very similar bond to a triple bond but also has a charge separation. This greatly enhances the reactivity of the molecule.
Now that we understand the structure of this molecule, we examine its use in the industrial world.
What are its uses, and how it is produced?
I want to immediately clarify a concept: the carbon monoxide is an essential molecule for the modern reality. The latter, together with molecular hydrogen (H2), form a very dear to the chemical mixture called syngas. Syngas, or synthesis gas, is to be the starting point for many of the worlds synthesis of organic and inorganic industrial chemicals.
Consider, for example, that starting from carbon monoxide and molecular hydrogen is synthesized methanol. The methanol, in turn, is involved in the synthesis of many compounds of high importance (acetic acid, formaldehyde, …), and these compounds in turn generate many materials that characterize our daily life. In short, its importance is high.
How to produce the syngas? In industrial practice, there are several possible ways:
Steam Reforming (SR): This reaction involves the interaction between the hydrocarbon molecules and water, to form CO and H2. Considering the simplest hydrocarbon species (methane), the reaction is as follows:
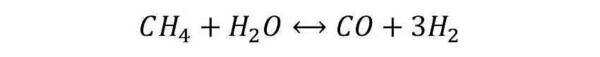
The reported reaction is extremely endothermic (requires a lot of heat). For this reason it is carried out in suitable reactors, in which are present the burners that heat the tubes by radiation and convection.
Partial Oxidation (OP): The species hydrocarbon, if subjected to a total oxidation process, tend to produce water (H2O) and carbon dioxide (CO2). The amount of oxygen required to make happen this process is called stoichiometric amount. If the combustion process is fed an amount of oxygen less than the stoichiometric, the hydrocarbon species will not have to meet to total oxidation, and therefore will suffer a partial oxidation process, which generates CO and H2. Considering again the methane, the stoichiometry of the process is the following.
This reaction, in contrast to that previously described, is found to have a markedly exothermic character (gives off a lot of heat)
Autothermal reforming (RA): The Steam Reforming and Partial Oxidation, as seen above, respectively endothermic and exothermic character. Is obvious question: why not use the heat generated by the second reaction to be first? On the basis of this demand we have developed the autothermal reforming, which uses the previously described reactions to produce the syngas.
Well, so far we have examined the context in which the carbon monoxide production was desired. There are many contexts, involving mainly combustion processes, in which the CO production is undesired, and in which this species can be seen as a real pollutant species.
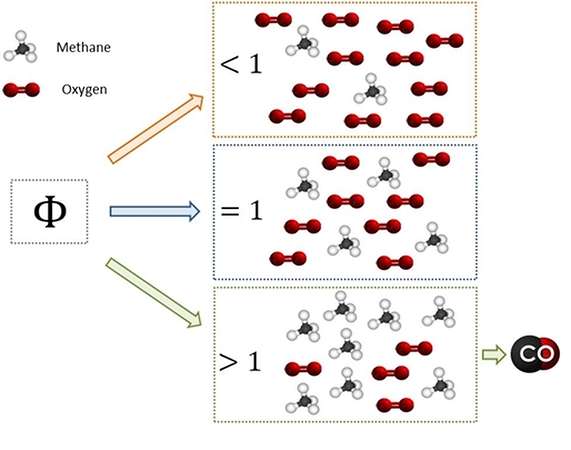
How come the production, in these contexts? In a previous article, devoted to the TAR, we introduced the concept of an equivalent ratio (comparing the relative amounts of fuel and air in the operating conditions
and in the stoichiometric conditions). We had also seen that, while feeding an adequate content of air (useful for complete combustion), in consequence of the inefficiency of mixing phenomena can arise with excess of fuel areas and the areas with a shortage of fuel.
In areas with excess of fuel it is impossible the achievement of the complete combustion condition, and it will then form of partial combustion products (such as, precisely, the CO).
We are ready to examine the CO harmful effects on human beings and how to intervene, in order to reduce the formation or to bring it down.
What effects does it have? How to intervene?
Effects
Carbon monoxide appears to be a highly toxic compound, whose inhalation can lead to unconsciousness and death.
What makes this compound so dangerous to humans? To explain this, we consider the phenomenon of respiration.
The oxygen that enters our lungs is then transported around the body thanks to red blood cells
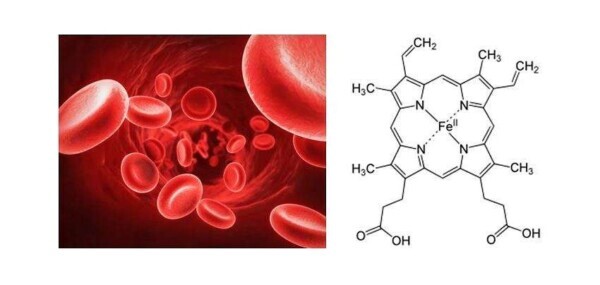
Within the red blood cells is a chromoprotein called hemoglobin, which in turn contains the heme groups (represented in the figure to the right). The function of these groups appears to be of extreme importance in the breathing process. Their role is to coordinate the red blood cells the oxygen molecules introduced through respiration, and thus provide for their transportation within the blood system. Without this process, our cells would not provide the necessary oxygen for their metabolism.
What happens when we breathe in carbon monoxide? Also this molecule is able to bind to the heme group of red blood cells, but so much stronger than the molecular oxygen molecules. In fact, while the link between red blood cells and molecular oxygen has reversible nature (and thus it allows the release of oxygen in the same of necessity) areas, the link between red blood cells and carbon monoxide leads to the formation of carboxyhemoglobin, a very stable complex. In this way it undergoes a rapid saturation of the available red blood cells, and to a resulting loss in oxygen transport capacity.
As previously said, to significant concentrations of CO, this process can lead to loss of consciousness and death by anoxia.
How to intervene?
So, how do we intervene? How it can affect the processes of formation of carbon monoxide, or remove it from gas streams when it has already occurred with the executive summary?
First we can reason in advance. We have seen earlier that, in the combustion process, the carbon monoxide is synthesized as a result of the mixing inefficiencies. A deficient mixing at the molecular level may lead to the genesis of rich fuel zones: in these areas there is not enough oxygen to promote complete oxidation of the hydrocarbon molecules, resulting in the formation of partial combustion products.
How to intervene? On the basis of what said, the answer is simple: to promote mixing.
This process can be effected by a thermal oxidation chamber, containing sometimes a suitable catalytic system that converts the molecules partially oxidized into their corresponding oxidized. In the automotive world it tends to use the appropriate catalytic converters, which operate by means of suitable transition metals and promote the conversion of CO to CO2.
See you soon with new and interesting articles!